Plants absorb nitrogen more than any other chemical. As such, this nutrient is absolutely crucial to their survival alongside phosphorus and potassium.
Nitrogen is vital to the development of proteins in plants. This allows them to photosynthesize and develop strong cell walls and healthy growth. They get nitrogen through the nitrogen cycle, a naturally-occurring system regulated by the environment. They also get nitrogen through fertilizers.
The nitrogen cycle is a fascinating process that sustains most plant life on the planet. Read on to learn more about how we utilize it for farming and the extra measures we take to nourish plants using fertilizers.
Why Nitrogen Is Essential for Plants
Every plant needs a constant supply of nutrients to remain healthy. As noted earlier, nitrogen is one of the most vital among these nutrients.
Nitrogen comprises part of chlorophyll, which is the lifeblood of a plant. Without it, the plant cannot photosynthesize. If the plant manages to grow, it’ll be weak and won’t produce fruit and flowers as it’s supposed to.
In fact, nitrogen makes up part of the nucleic acids DNA and RNA. These are the keystones of life and carry genetic information, instructing living things on how to…well, live.
That’s why nitrogen is so essential for commercial farming. If crops and fruit fail, the farmer has nothing to subsist on.
Nitrogen deficiency is somewhat less devastating for the everyday plant enthusiast. However, it’s still disheartening to see a plant struggle and wilt, especially if you don’t know what’s causing the plant to suffer.
What Is the Nitrogen Cycle?
Earth’s atmosphere comprises around 80% nitrogen, but humans and other living things can’t absorb this essential chemical directly from the air.
The nitrogen cycle is how nitrogen moves through the environment and eventually reaches the living things that need it to survive. Plants absorb nitrogen through the soil, animals eat these plants while humans eat both plants and animals.
Nitrogen fixation, or fixing, is the process whereby atmospheric nitrogen converts into nitrogen (nitrate) in the soil.
The nitrogen cycle consists of five key steps. These turn nitrogen into a usable form and include:
- Nitrogen fixation
- Mineralization
- Nitrification
- Immobilization
- Denitrification
Let’s take a look at each of these steps in-depth to better understand the processes that sustain all living things.
Nitrogen Fixation
Nitrogen is gaseous, thus plants can’t use it directly from the atmosphere. Nitrogen fixation is the process that converts nitrogen to nitric acid. Various natural processes enable this conversion.
As bizarre as it sounds, lightning can create chemical reactions in the atmosphere, turning nitrogen into nitrous oxide. This mixes with rainwater to become nitric acid, increasing the amount of nitrate in the soil.
The process occurs naturally for the most part, but burning fossil fuels also releases nitrous oxide into the atmosphere. This may sound like a good thing, but it isn’t.
Human interference upsets the earth’s natural ecology and throws off the delicate natural balance that keeps all living things in check.
Mineralization
After nitrogen has converted to nitric acid in the soil, it undergoes another chemical process while inside the earth.
Microbes act on organic material in the soil. The organic material includes the following:
- Animal excrement: Bat guano and cow excrement are also commonly used in manure and natural fertilizers.
- Decaying animal matter: Organic fertilizers sometimes come in the form of ground-up fish bones and another natural, decomposed material.
- Decaying plant matter: Using dead leaves and wood in compost boosts its nutritional value immensely and encourages rapid plant growth.
The organic material returns nitrogen to the soil as ammonia. This becomes nitrite, and nitrifying bacteria then convert this to nitrate through a process called mineralization.
Take a look at the below video for a deeper explanation of how mineralization works in regards to crop yield:
Nitrification
In this stage, the ammonia released by organic matter becomes nitrate. Plants contain nitrifying bacteria that convert nitrogen to a form they can absorb by harnessing oxygen in the soil.
There are two kinds of bacteria involved:
- Nitrobacter converts nitrites into nitrates.
- Nitrosomonas convert ammonia to nitrates.
Nitrification and denitrification are basically opposites. Nitrification converts nitrogen from the atmosphere into a form plants can absorb. However, denitrification turns the nitrogen in the soil to a gaseous state, returning it to the atmosphere.
Immobilization
Microorganisms in the soil soak up nitrates and ammonium present in the ground. This limits the amount of nitrogen that plants can absorb through the soil. In a way, this is the opposite mineralization.
If this process is unbalanced, plants may become nitrogen deficient. However, in a balanced process, these microorganisms are essential in immobilizing the nitrogen in the soil so plants don’t take in excess nitrogen.
Bacteria decide whether they’ll mineralize or immobilize nitrogen based on particular conditions, which are discussed in the video below by Pen State Extension.
This video demonstrates how intelligent our planet’s natural processes genuinely are, creating a chain reaction to every situation and balancing their environment based on every possible input.
Denitrification
In the final stage of the nitrogen cycle, nitrogen returns to the atmosphere. Bacteria denitrify nitrogen in the soil by oxidizing it.
The bacteria, also called heterotrophic bacteria, convert ammonium to nitrate and finally to nitrogen gas.
This nitrogen bubbles to the surface, water, or moisture in the soil, evaporating into the atmosphere, completing the nitrogen cycle and starting it anew.
Science Learning Hub provides a practical explanation of how denitrification works and what conditions need to be present for this process to occur.
How Do They Get the Nutrient?
Plants need a steady supply of nutrients and water to survive, one of the most important of which is nitrogen. We now know that plants absorb nitrogen through the soil instead of directly from the atmosphere, but this isn’t the only way plants get nitrogen.
Commercial fertilizers contain many chemicals and nutrients that allow plants to flourish, which are absolute staples in commercial farming. These fertilizers boost crop production, increase yield and quality, and make for overall healthier plants and crops.
Commercial fertilizers contain nitrogen in several forms:
- Ammonia: Ammonia is present in the atmosphere in a gaseous form or in water as a liquid called anhydrous ammonia.
- Ammonium: Ammonium results from a chemical reaction between ammonia and water. Ammonium attaches to organic matter, so it doesn’t leach, and microorganisms convert ammonium to nitrate for plants’ absorption.
- Nitrate: Nitrate dissolves in water and flows with the water in the soil. If the soil gets too much water, it can lead to leaching. On the other hand, too little water can cause nitrate to accumulate on the soil’s surface.
- Urea: Enzymes present in the soil convert urea to ammonia and then to ammonium. Other bacteria convert this ammonium to nitrates that dissolve in water.
Leaching is a considerable problem in farming and general plant care. Leaching is when nitrogen gets washed deep into drainage systems within the soil, away from where plants can reach and absorb it.
In addition, nitrogen can drain through the soil and end up in groundwater or surface water. This is a considerable health risk to people who may consume this water.
A solution to this is injecting nitrogen into the soil. No-till opening coulters inject liquid nitrogen just below the ground’s surface, significantly decreasing the risk of nitrogen loss through leaching.
The main drawbacks of this method are that it takes far more time and money than just using regular nitrogen-rich fertilizer. In most cases, injection comes out on top as the superior method, but in some cases, the results are equally effective.
Because of this, there’s a case to argue for surface banding (applying fertilizer to the soil’s surface along crops). In cases of arid, hot weather, the surface application is at a much higher risk of failing, and nitrogen injection is a far better option.
Nutrients in Commercial Fertilizers
Commercial fertilizers contain the following main ingredients which are vital to growing plants and crops successfully:
- Nitrogen
- Phosphorous
- Potassium
- Zinc and other metals, or micronutrients.
Fertilizers can be made in a variety of ways. You can use:
- Raw materials
- Compost
- Manure
- Industrial waste
- Sewage waste
Sewage and biowaste make excellent fertilizers. They contain a wealth of nutrients and metals that aid in plant growth.
Recycling industrial wastes, on the other hand, can result in many heavy metals in the soil. This is why they need to be tested for their micronutrient concentrations and be correctly processed and applied to make a positive difference.
Consequently, using this method for agricultural purposes requires a special permit from the EPA Regional Administrator because the risk of human illness is far greater with consumable plants.
Choosing a Fertilizer
Deciding on a suitable fertilizer requires an intimate knowledge of the following:
- The soil you’re using.
- The current environmental conditions.
- The pH your specific trees or plants need to grow.
You should also consider which kind of fertilizer you’d like to use:
- Synthetic/traditional fertilizer
- Organic fertilizer
The table below lays out the main features of both fertilizer types.
| Fertilizer | Description | Price | Application | Environmental risk |
| Synthetic | Made from inorganic materials. | These are more commonly used commercially and are cheaper. | They’re water-soluble and give your plants a quick boost. Results begin to appear within one to two weeks after application. | Applying excessive amounts can result in fertilizer burn, and this type has a much greater chance of leaching. |
| Organic | This type uses organic materials and poses no risk of fertilizer burn. | These aren’t used as much commercially and cost more than synthetic fertilizers. | Organic fertilizer takes longer to apply and comes in more limited forms. It has a much slower release time. | They won’t cause damage if you apply too much, unlike synthetic fertilizers. |
This is a condensed explanation of the different kinds of fertilizers. The exact science of fertilizers is based on the ratio of nitrogen, phosphorus, and potassium found in the soil and which elements need to be supplemented most.
For a detailed explanation of the numbers and letters involved in fertilizer classifications, take a look at this article here.
Despite the higher price and longer application time, it’s overall healthier and more beneficial to use organic fertilizers rather than synthetic ones.
Using organic fertilizer gives plants the tools they need to strengthen themselves with naturally-occurring materials. This benefits your plants and soil in the long term. Furthermore, these fertilizers are made of materials that are easy for plants to absorb.
Because plants are given the strength to sustain themselves, they also become far more capable of fighting off pests and diseases. They can also regulate their moisture and nutrient intake effectively without interference.
The materials used in organic fertilizer include:
- Bone meal
- Feather meal
- Guano
- Fish carcass
- Soybean meal
- Cottonseed meal
- Compost
- Manure
Cesco Solutions Inc. Urea Fertilizer (available on Amazon.com) Is a brilliant fertilizer solution for indoor and outdoor plants of all kinds. The nitrogen-rich urea granules boost healthy growth and pose no health risk for the user or the plants.
Infinity Soil 4-17-0 Fish Bone Meal (available on Amazon.com) is super affordable and particularly rich in calcium, phosphorus, and nitrogen. This all-natural fertilizer is safe and effective for indoor and outdoor plants and consumable plants like vegetables and fruit trees.
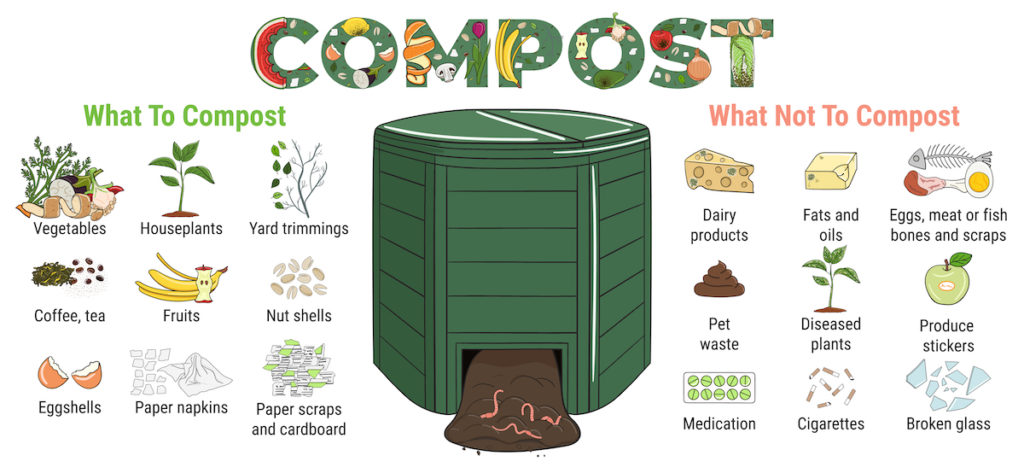
If these and other similar products aren’t quite what you’re looking for, you can endeavor to make your own compost. The process is cheap and straightforward but it does require a fair amount of patience, as compost can take up to two years to develop properly.
You can build up a compost heap or bin over time with everyday items, such as:
- Eggshells
- Banana peels
- Coffee grounds
- Fallen leaves
- Sawdust
- Untreated wood chips
- Fruit and vegetable scraps
Learn how to make your own compost from Better Homes & Gardens following a simple, step-by-step procedure.
If you opt for the synthetic fertilizer route, Miracle-Gro All Purpose Plant Food (available on Amazon.com) is an excellent bang for your buck. It’s jam-packed with all kinds of nutrients for all types of plants, including flowers and vegetables, and won’t cause any harm to your plants.
Alternatively, the Fox Farm Nutrients Soil Trio Liquid Plant Fertilizer (available on Amazon.com) is the trifecta of great fertilizer options. These guarantee green growth and healthy fruit and flower growth throughout the growing season.
The Negative Effects of Too Much Nitrogen
On the whole, nitrogen is vital to life on earth. But is it possible to have too much of a good thing?
While a balanced amount of nitrogen in the soil will sustain plant life, an excess of it will do the exact opposite.
- Chemical imbalance: The natural balance in the soil is delicate, and an excess of nitrogen will quickly deplete other essential nutrients like calcium and magnesium.
- Plant loss: Weeds have a considerable tolerance and need for nitrogen, so while they can flourish in the wake of excess nitrogen, indigenous, desirable plants will likely suffer and die. As a result, your garden will become overrun with weeds.
- Health risks: Leaching negatively affects plants and people. If nitrogen gets into drinking water in large amounts, people will develop gastrointestinal issues and other severe health issues, posing an exceptionally high risk to the very young and elderly.
Although nitrogen-rich fertilizer can cause more damage than good if misused, the effects of human negligence dwarf the adverse effects of fertilizer.
The burning of fossil fuels and the increased use of greenhouse gases have permanently altered our natural resources, from the sky to the seas. One of the effects of this is a higher rate of nitrogen production that the planet can’t keep up with and has to battle to balance out, resulting in considerable natural unrest.
The harmful effects of human interference on the planet affect both plants and animals. Still, they also have an enormous impact on human health and well-being, as contaminants cause more and more allergies and illnesses globally.
As nitrogen levels increase, ozone becomes more prevalent. According to National Academies, breathing ozone:
- Irritates the lungs. Common symptoms include shortness of breath and inflamed lungs, as well as a worsening of common allergies like pollen and dust allergies.
- Causes respiratory issues. Spikes in ozone levels open us up to more severe health issues like asthma, pneumonia, and chronic obstructive pulmonary disease (COPD) over long periods of exposure.
Read more on the adverse effects of nitrogen overproduction here.
Keeping Your Plants Healthy
Now that we know the ins and outs of nitrogen production, both natural and man-made, we need to understand how to effectively keep our plants nourished and healthy. Below are some helpful suggestions:
- Regularly test the nitrogen levels in your soil. Using nitrate test strips, ensure you routinely check the nitrogen levels in your soil, to maintain a healthy nutrient balance at all times. This will yield the most successful plant growth.
- Use the correct fertilizer. Check out how much nitrogen your plant needs before fertilizing it to ensure it’s getting the optimal amount. The temperature and time of year also play a significant role in this.
- Monitor watering habits. Water your plants regularly or as recommended, but ensure you don’t overwater your plants. Overwatering can quickly lead to leaching, which would move precious nutrients out of the reach of plants.
- Don’t neglect other nutrients. With all this talk of nitrogen, it’s easy to forget about the other nutrients. Use fertilizers that contain the three primary nutrients, but ideally also those that contain calcium, magnesium, iron, and other essential chemicals.
Summary
Nitrogen is the lifeblood of the planet. Though it makes up 80% of our atmosphere, it isn’t easily accessible to plants; nitrogen undergoes an endless transformation cycle to feed plant life.
We must try our best to reduce air pollution to maintain the natural balance and ecosystems, allowing them to self-regulate and produce enough nutrients to keep plants healthy and thriving.

